Colombia Cannabis Resources + Regulations
Colombia Approves Cannabis Flower for Export
Updated: January 2023
The growing environment in Colombia is ideal for large-scale outdoor cultivation, leveraging their rich agricultural experience. With 12 hours of sun year-round affording multiple cannabis harvests, low labor costs, and cheap land, Colombia is positioned to be one of the world’s low-cost suppliers.
Colombia legalized cannabis in 2016 for the export of low THC oils and extracts, and gained further international attention when Decree 811 approved the export of dry cannabis flower in July 2021. However, it wasn’t until April 1, 2022 when Resolution 539 when actual regulatory mechanisms went into effect, allowing for the export of dried flower with high THC levels.
With this policy change, Colombia is positioning itself to become a key low-cost supplier to the global medicinal cannabis market.
Consequently, international investment has continued to expand, with the purpose of exporting to the European Union, Israel, and Australia. More than 18 multinationals have set up shop in Colombia.
Export Markets Drive Colombia’s Quality Standards
The European Union – with a chronic undersupply of local cultivators – is arguably the largest export opportunity for medicinal cannabis – and Colombia. As the market evolves, European administrators are pursuing a regulatory framework modeled on the pharmaceutical industry.
Colombian growers seeking to export into the EU’s 27 countries and 350 million residents must follow EU GACP (Good Agricultural and Collecting Practice) and GMP (Good Manufacturing Practice) guidelines for growing and manufacturing, respectively, as well as microbial limits established by the European Pharmacopoeia.
And here the picture begins to get murky. The existing European Pharmacopoeia was never intended to address all the formulations and characteristics of medicinal cannabis. Moreover, there is no EU monograph for cannabis. Consequently, there is a wide range of interpretation on which Ph. Eur. Standards should be followed for exporting into the EU when it comes to microbial compliance.
The below table summarizes the range of microbial requirements under 5.1.4 and 5.1.8, the two standards most often cited for microbial compliance.
The efficacy of radio frequency is an effective solution to meet stringent Ph. Eur standards.
| Ph. Eur. 5.1.4.1 | Ph. Eur. 5.1.4.2 | Ph. Eur. 5.1.8 – Table B | Ph. Eur. 5.1.8 – Table C |
|---|---|---|---|
| Acceptance criteria for microbiological quality of non-sterile dosage forms | Acceptance criteria for microbiological quality of non-sterile substances for pharmaceutical use | Herbal medicine products with or without recipients, where the method of processing (pre-treatment) reduced the levels of organisms | Herbal medicine products with or without recipients, where the method of processing (pre-treatment) cannot reduce the level of organism to table B requirement |
| TAMC <100 CFU/g TYMC <10 CFU/g BTGN BTGN not detected in 1g Pathogenic E.coli not detected /g Salmonella not detected /g S. aureus not detected /g P. aeruginosa not detected /g | TAMC <1,000 CFU/g TYMC <100 CFU/g BTGN not detected in 1g Pathogenic E.coli not detected /g Salmonella not detected /g S. aureus not detected /g P. aeruginosa not detected /g | TAMC <10,000 CFU/g TYMC <100 CFU/g BTGN <100 CFU/g Pathogenic E.coli not detected /g Salmonella not detected /g | TAMC <100,000 CFU/g TYMC <10,000 CFU/g BTGN <10,000 CFU/g Pathogenic E.coli not detected /g Salmonella not detected /g |
The lack of a harmonious regulatory regime in the EU is detailed extensively by German regulatory expert Markus Veit “Quality Requirements for Medicinal Cannabis in the European Union – Status Quo” March, 2022.
German Import Requirements Favor Radio Frequency
Germany specifically has further complicated matters for exporters, requiring registration of all strains remediated with ionizing radiation (gamma, e-beam, x-ray) prior to distribution in Germany. Registration takes anywhere from 12-18 months, and an administrative fee of €5,000 – per strain. As strains are continually evolving to meet market demand, this is a costly barrier to doing business in Germany.
The following excerpt from Novacana GmbH, a German importer, summarizes the situation:
“The regulatory regime overseeing the distribution of medicinal cannabis in Germany is continuously changing. Cannabis flowers are usually treated with ionizing radiation in order to protect them permanently against bacteria and mold and reduce germ count. However, according to the Medicines Act (AMG), pharmacies are prohibited from selling medicinal products that have undergone ionizing radiation unless they have been approved in accordance with the Order on radioactive medicinal products and medicinal products treated with ionizing radiation (German designation: AMRadV, Irradiation Decree)
Since late 2019, this was eventually being enforced on imported cannabis flowers to Germany from the Netherlands and Canada as well. Medicinal Cannabis flowers cultivated under an EU-GMP regime are routinely irradiated with gamma waves to reduce the bacterial and fungi count and increase shelf life. Unless the distribution complies with the Irradiation Decree (AMRadV), German law forbids the distribution of irradiated product. This regulation stipulates that the distributor must hold such a permit when supplying these products to other wholesalers or pharmacies.
The submission for this license is extensive and necessitates elaborate information on the manufacturing process, validation equipment as well as risk evaluation to stability data of the irradiated product.”
Radio Frequency is an optimum solution to the AMRadV requirement, as RF is a non-ionizing thermal process and thus provides a streamlined pathway for export into Germany, reducing time, money, and administrative expense.
Business Case For Using Radio Frequency
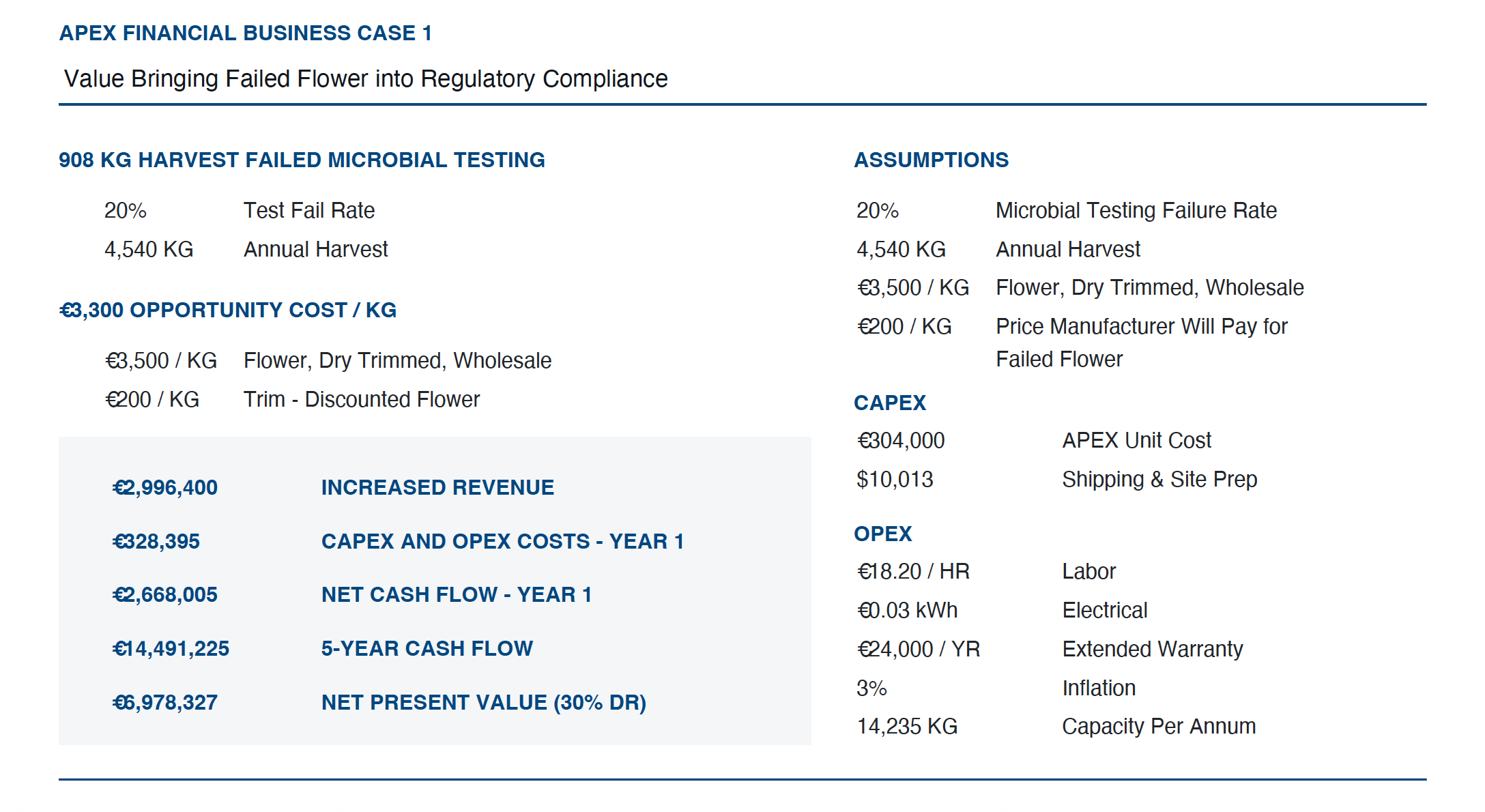
Ziel’s APEX Business Case allows customers to input their own assumptions and determine how much money APEX 7 will save their business from day one remediating. The model looks at the percentage of product currently failing and bringing that into regulatory compliance, versus having to discount failed product by up to 90% and selling as trim to a manufacturer.
In the example below, we use a wholesale price of $1,200 with failed product discounted to $100 and sold as trim. The snapshot below shows the savings a cultivator will accrue within the first year using the APEX 7, based on recovering 20% of the harvest that fails microbial testing on 10,000 lbs of dry flower harvested per annum.
APEX also has the largest throughput of any technology on the market today, allowing you to scale your business operations.
Clean cannabis technology is a vital safeguard as regulations are only becoming stricter. Ensuring your harvest passes microbial testing increases your top-line revenue, and optimizes your financial returns, improving your bottom line. Incorporating remediation into your post-harvest SOPs streamlines operations and improves process flow by avoiding costly retesting and gets your product out the door quicker to customers.
About Ziel
Ziel is a leading developer of Radio Frequency (“RF”) solutions for the reduction of microbial pathogens. The food and cannabis industries across North America, Europe, South America, and Australia rely on RF technology to safely remediate products intended for human consumption or ingestion. Ziel’s devices utilize non-ionizing radiation to pasteurize products like almonds, cashews, macadamias, sesame, and chia.
The RF technology has been adapted for the cannabis industry to successfully remediate bacterial and fungal pathogens. These devices help cannabis cultivators ensure that they are providing a safe product that meets the highest safety and quality standards. Moreover, Ziel’s technology allows licensees to satisfy these standards through a method that is compatible with the requirements for organic certification.
Contact Ziel to learn more about our innovative cannabis processing system for Colombia-based growers.